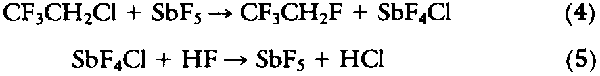Reproduced, with permission, from:
Manzer, L. E. 1990. The CFC-ozone issue: Progress on the development of alternatives to CFCs. Science 249: 31-35.
Reproduced, with permission, from:
Manzer, L. E. 1990. The CFC-ozone issue: Progress on the development of alternatives to CFCs. Science 249: 31-35.
The CFC-Ozone Issue: Progress on the Development of Alternatives to CFCs
L. E. MANZER*
Chlorofluorocarbons (CFCs) are now believed to be major contributors to the seasonal ozone depletion over the Antarctic continent. However, because they are so important to many aspects of modern society, it would be irresponsible to immediately cease their production. The identification of suitable substitutes is difficult when issues such as toxicity, flammability, cost, environmental impact, and physical properties are considered. Several candidates that meet these criteria have been selected by the industry and significant research and development programs are under way to commercialize them. Unlike the simple, fully halogenated CFCs, which can only be made in the single step, there are many potentially viable routes to the alternatives, but these will require significant improvements in catalysis. Many other important issues such as materials compatibility, energy efficiency, the needs of developing countries, and the product life cycle of the alternatives need to be resolved before a timely transition to substitutes can be accomplished.
THE DEVELOPMENT OF FLUOROCARBON CHEMISTRY WAS pioneered by Swarts during the late 1890s (1). He found that C-F bonds could be formed by the stoichiometric reaction of SbF3 with activated C-Cl bonds, but the applicability of the reaction was limited:
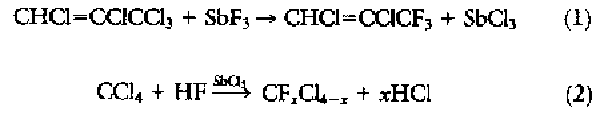 When Swarts later discovered
that the addition of trace quantities of pentavalent antimony as a "fluorine
carrier" allowed the reaction to be extended to a wide variety of chlorocarbons
(2), the era of commercially attractive fluorocarbon
chemistry began. The significance to modern society of fluorocarbons in refrigeration
systems, however, was not recognized until more than 30 years later.
When Swarts later discovered
that the addition of trace quantities of pentavalent antimony as a "fluorine
carrier" allowed the reaction to be extended to a wide variety of chlorocarbons
(2), the era of commercially attractive fluorocarbon
chemistry began. The significance to modern society of fluorocarbons in refrigeration
systems, however, was not recognized until more than 30 years later.
Early refrigeration systems were developed with cooling fluids such as CH3Cl, SO2, NH3, C2H2Cl2, CH2Cl2, and hydrocarbons. From a thermodynamic standpoint, they gave good refrigerating performance but were flammable and toxic. Several years later, in 1928, two scientists at the Frigidaire Division of General Motors were asked to develop nonflammable, nontoxic replacements for these hazardous cooling fluids in home refrigerators. Within 2 days, Midgley and Henne had selected CFC-12 (CCl2F2) as an ideal refrigerant (3), and a joint venture between General Motors and Du Pont was formed to commercialize this complex technology.
The initial stoichiometric reaction between the SbCl2F3 and CCl4 was developed into a continuous process whereby the CCl4 and HF were continuously fed to a reactor containing pentavalent antimony (2). By adjusting process conditions, the degree of fluorination on the carbon could be controlled, and as a result a wide variety of new compounds was introduced into the market over the next 50 years. This synthetic route forms the basis for modern-day commercial CFC processes for the manufacture of CFC-11 (CFCl3), CFC-12 (CF2Cl2), HCFC-22 (CHF2Cl), CFC-113 (CF2ClCFCl2), CFC-114 (CF2ClCF2Cl), and CFC-115 (CF3CF2Cl) (Table 1).
By 1988, the total world consumption of CFCs had grown to over 10[9] kg. In the United States some 5,000 businesses at nearly 375,000 locations produce CFC-related goods and services worth more than $28 billion a year (4). CFC-related jobs total more than 700,000. Within the United States, the three major uses of CFCs are as refrigerants (30%), as foam-blowing agents for polystyrene and polyurethane (28%), and as industrial solvents and cleaning agents (19%). Outside North America, a significant amount (>1.5 x 10[8] kg) of CFCs have continued to be used as aerosol propellants, even though that application was essentially banned in the United States in 1978. Conversion of CFCs for aerosol applications could occur rapidly (the technology has been developed over the past 10 years in the United States).
The chemical stability of the CFCs that leads to their desirable safety characteristics also contributes to their ability to deplete atmospheric ozone (O3) (5). There are no known destructive mechanisms for CFCs in the troposphere. Thus, once released, they rise to the stratosphere and are decomposed by solar ultraviolet radiation. The first real evidence of possible concern was Lovelock's measurement of the atmospheric concentration of CFC-11 (6) in the early 1970s. A comparison of estimated releases of the compound with its concentration indicated that very little, if any, had decomposed and thus that the stable CFCs were accumulating in the atmosphere.
The first reaction to this information was a Du Pont-organized "Seminar on the Ecology of Fluorocarbons" for the world's CFC producers in 1972. The invitation, written by Ray McCarthy, said: "Fluorocarbons are intentionally or accidentally vented to the atmosphere worldwide at a rate approaching one billion pounds per year. These compounds may be either accumulating in the atmosphere or returning to the surface, land or sea in the pure form or as decomposition products. Under any of these alternatives it is prudent that we investigate any effects which the compounds may produce on plants or animals now or in the future." As a result of that industry symposium, a research program sponsored by 19 companies was established to investigate the fate and impact of CFCs in the atmosphere.
Lovelock's measurements also initiated the research of Molina and Rowland into the atmospheric fate of CFCs (7). Their conclusion was that CFCs in the stratosphere would be photochemically broken down, releasing chlorine atoms, which then would enter into a catalytic cycle involving the destruction of O3. Because stratospheric science was in its infancy at that time, however, there was no reliable means to check the validity of the O3 depletion theory. By the mid-1980s, the growth in the use of CFCs in refrigeration, cleaning agents, and foam insulation markets more than offset the decline in the use of CFCs in aerosol markets in the United States and Canada. Furthermore, forecasts projected continued demand, due largely to the recognition that developing countries would require the services provided by CFCs. These growth forecasts, coupled with improved understanding of the potential for O3 depletion if CFC emissions increased, led to international efforts to limit long-term growth of CFC emissions.
Intense discussions continued through September 1987, when a historic international agreement, the Montreal Protocol, was signed. It called for a 50% reduction of CFC production by the year 1998 and periodic reviews of the science.
The ratification process for the protocol had just begun when the National Aeronautics and Space Administration Ozone Trends Panel announced new findings (8), which raised serious questions about whether the restrictions on CFC production and use contained in the protocol were adequate to protect stratospheric O3. Because of this consensus linking CFCs with O3 depletion, Du Pont and other companies announced a total phaseout of CFC production by no later than the end of the century, and CFC-producing companies initiated research and development programs to develop environmentally safe substitutes. It now seems likely that the first review of the Montreal Protocol, in mid-1990, will also call for a global phaseout by the year 2000, with a 10-year lag time for developing countries.
Selection of Appropriate Substitutes
A recent market projection by Du Pont has attempted to determine how the current markets that use CFCs will be satisfied in the year 2000 (9) (Fig. 1). Increased awareness of environmental issues and cost will result in about a 30% reduction in the market for CFCs through improved conservation measures, including better maintenance systems and product recovery, recycling, and reclamation.
Another 30% of the market will switch to less expensive, not-in-kind replacements. For example, systems based on organic compounds and H2O may be used for certain cleaning applications, and CO2, hydrocarbons, and water could be used as foam-blowing agents. Du Pont, however, predicts that the remaining 40% of the projected market will still require fluorocarbon-based products because of the unique properties associated with these molecules, particularly in the refrigeration industry. The low vapor-phase thermal conductivity of CFCs contributes to the efficiency of plastic insulating foams for refrigerators, freezers, buildings, and refrigerated railway cars and trucks. Even if the foams could be expanded with air or CO2, the thermal efficiency would be reduced by about a factor of 2. The energy penalty associated with the elimination of hydrochlorofluorocarbons (HCFCs) from insulating foams could be equivalent to several billion gallons of additional fuel consumed in the United States annually. Hydrofluorocarbons (HFCs) and HCFCs have emerged as alternative fluorocarbon products for the remaining market segment and will form the basis for the rest of this discussion.
Selecting appropriate product substitutes for an industry that has developed over 50 years is no trivial task. Many factors must be considered and thoroughly evaluated. Foremost is environmental acceptability for the O3 and other environmental concerns such a global warming and acid rain.
One can calculate the O3 depletion potential (ODP) of a compound by dividing the cumulative calculated O3 depletion caused by the release of a compound by the calculated O3 depletion of an equal emission (by weight) of CFC-11. [In a similar manner, the relative halocarbon global warming potential (halocarbon GWP) can be calculated.] ODP is an estimate of relative effects and should therefore be used to determine the relative long-term environmental benefits of alternative compounds. Because the HFCs do not contain chlorine and therefore have an ODP of zero, they are very attractive alternatives. The absence of chlorine, however, often gives HFCs a higher vapor pressure and lower solubility than CFCs. These two characteristics limit their use for some applications. Significantly reduced ODPs can be obtained by introducing hydrogen atoms into the molecule, even if it contains chlorine. The presence of chlorine causes the molecule to decompose in the troposphere so that very little of the material reaches the O3 in the stratosphere. Options other than these HCFCs have not been identified for energy-efficient insulating foams, cleaning agents for critical electronic and metal components, and certain refrigeration and air-conditioning applications.
The estimated $135 billion worth of equipment in the United States that depends on CFCs has an expected lifetime of 20 to 40 years. Therefore, it is important that the physical properties of an alternative closely match those of the CFC they are replacing. If one considers C1 to C3 molecules that contain only fluorine, hydrogen, and chlorine as substituents, over 88 generic formulas are possible. Regiochemistry can have a significant effect on physical properties. For example, HFC-143a (CF3CH3) and HFC-143 (CF2HCH2F) have boiling points of -48deg. and +5deg.C, respectively. If all isomers are included, the list grows to 601. Fortunately, this large number of candidate compounds can be reduced by the use of a simple qualitative technique devised by McLinden and Didion, which deletes molecules based on projected flammability, toxicity, and atmospheric lifetime (10). Using this qualitative approach, one can narrow down the choices considerably so that the challenge then becomes one of selecting molecules from those that remain on the list that have the desirable physical properties.
Vapor pressure curves as a function of temperature have been determined for a wide variety of HFCs and HCFCs (11). The most attractive are shown in Fig. 2. Clearly, HFC-134a (CF3CH2F), HCFC-123 (CF3CHCl2), and HCFC-141b (CH3CFCl2) are the closest match to CFC-12 and CFC-11 over a wide temperature range. However, on the basis of a comparison of HFC-134a and CFC-12, it can be seen that the match is not perfect at the high and low end of the vapor pressure curves, so that a redesign of the current refrigeration system will be required.
Many of the other compounds in Fig. 2 may also have potential utility in specialty applications; however, that evaluation can only occur after developmental quantities become available. In many cases, enhanced performance may be obtained if two or more compounds are combined as blends or azeotropes. For example, Du Pont has announced a family of blends that may prove to be near "drop-in" replacements for CFC-12 (12). Two blends were highlighted, consisting of HCFC-22 (CF2HCl), HFC-152a (CHF2CH3), and either CFC-114 (CF2ClCF2Cl) or HCFC-124 (CF3CHFCl). The latter composition has an ODP that is only 3% of CFC-12. These blends do not appear to have the problems with oil compatibility in automotive air-conditioners, as does HFC-134a, and may be very attractive substitutes for systems equipped to use CFC-12. It is estimated that 60 million vehicles designed to use CFC-12 will still be operating in the United States in the year 2000.
An important molecule that has unique properties as a solvent and cleaning agent is CF2ClCFCl2 (CFC-113). Much research has focused on the identification of an acceptable single substitute, but without much success. Asahi Glass has announced the development of two candidates, HCFC-225ca (CF3CF2CHCl2) and HCFC-225cb (CFHClCF2CF2Cl). The boiling points of these two compounds are in the right range, compared to CFC-113. Asahi Glass expects to commercialize these compounds in 4 to 5 years. Here again, azeotropes and blends may find applications. Blends of HCFC-123 and HCFC-141b in CH3OH have been announced and offer excellent properties. Two hydrocarbon-based products that contain no halogens have been introduced for semiaqueous cleaning systems. These potential products are examples of the not-in-kind replacements discussed earlier.
In summary, the most likely substitutes for the three major CFCs are shown in Table 2. It should be pointed out that CHF2Cl (HCFC-22) has been produced for many years and is currently used in certain foam-blowing and refrigeration applications. Additional growth in the use of this compound will likely continue. The projected costs for these molecules are approximately two to five times those of the CFC they are replacing as a result of the complexity of new manufacturing processes and higher ingredient costs attributable to the higher molecular weights of the substitutes.
Potential Routes to CFC Alternatives
The only viable chemical route to the simple CFCs involves the reaction of a chlorocarbon with HF, either in the gas phase over heterogeneous catalysts or in a liquid phase, as illustrated in Eq. 2. For the two-carbon CFC alternatives, the chemistry becomes much more complicated, because there are now at least four chlorocarbons that could be produced on a large scale commercially. A large number of routes can be visualized to convert each of these feedstocks to HFC-134a (Fig. 3). Two examples will illustrate the complexity of this chemistry.
CF3CH2Cl (HCFC-133a) routes to HFC-134a. HCFC-133a is a small-volume commercial product today and is carcinogenic and embryotoxic. Its preparation is well documented either in gas-phase (13) or liquid-phase (14) reactions by the reaction of HF with trichloroethylene (TCE):
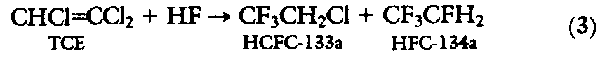 It appears unlikely that
this chemistry will provide a single-step, high-yield route to HFC-134a, analogous
to that for the preparation of CFC-12. Early work at Dow showed that the direct
reaction of TCE with HF gave only 3% HFC-134a and 78% HCFC-133a, with the use
of an "oxygenated chromium fluoride" catalyst at 300deg. to 400deg.C (15).
By increasing the temperature to 500deg.C over a modified Cr[6+]/Al2O3 catalyst,
Kaiser reported a 20% single-pass yield to HFC-134a (16).
The main products were HCFC-133a (50%) and 20% "other." Conversion of a -CH2Cl
group, such as that in HCFC-133a, to -CH2F is known to be a very difficult transformation,
often requiring expensive fluorinating agents (17).
Therefore, most work has focused on the high-temperature fluorination of HCFC-133a
with heterogeneous catalysts.
It appears unlikely that
this chemistry will provide a single-step, high-yield route to HFC-134a, analogous
to that for the preparation of CFC-12. Early work at Dow showed that the direct
reaction of TCE with HF gave only 3% HFC-134a and 78% HCFC-133a, with the use
of an "oxygenated chromium fluoride" catalyst at 300deg. to 400deg.C (15).
By increasing the temperature to 500deg.C over a modified Cr[6+]/Al2O3 catalyst,
Kaiser reported a 20% single-pass yield to HFC-134a (16).
The main products were HCFC-133a (50%) and 20% "other." Conversion of a -CH2Cl
group, such as that in HCFC-133a, to -CH2F is known to be a very difficult transformation,
often requiring expensive fluorinating agents (17).
Therefore, most work has focused on the high-temperature fluorination of HCFC-133a
with heterogeneous catalysts.
The reaction of HF with HCFC-133a requires a large excess of HF to drive it to a reasonable organic conversion. For example, a 30% single-pass yield of HFC-134a is obtained at 350deg. to 400deg.C with 6 to 10 mol of HF per mole of HCFC-133a fed (18). This requires a large recycling of organic and HF. Catalyst lifetime appears to be a major problem with this process. Daikin indicated that, with chromium-based catalysts, the conversion decreased 15% in only 40 hours of operation (19). However, by cofeeding small amounts of air, it was possible to maintain a constant conversion for 80 hours of operation. A recent patent suggests that a variety of metals on an aluminum fluoride support provide extended catalyst life (20). Another problem with this chemistry is that HF is eliminated from HCFC-133a, to give HCFC-1122 (CF2 = CHCl) at the high temperatures required for this reaction. The halogenated olefins are toxic and need to be removed from the HFC-134a. Selective removal by treatment of the crude HFC-134a with aqueous potassium permanganate or the low-temperature addition of HF to the olefin have been reported (21).
The unfavorable reaction of HCFC-133a with HF can be avoided by the use of KF as a stoichiometric reagent in either protic or nonprotic solvents (22). Although high temperature and pressures are required, the yield is good. Recent patents have also addressed the equilibrium limitations of the reactions with stoichiometric amounts of SbF5 used as a fluorinating agent (23):
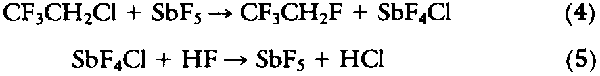
High conversions of HFC-134a were reported, but the process requires the extra step of regenerating the SbF5.
CF3CFCl2 (CFC-114a) routes to HFC-134a. Another two-step route that has received considerable attention in the patent literature involves CFC-114a as an intermediate:

Because only the asymmetric isomer, CFC-114a, is readily hydrodechlorinated to HFC-134a, a high isomer yield is important. Montedison has reported that the chlorofluorination of ethylene or tetrachloroethylene over promoted aluminum fluoride catalysts gives 75% CFC-114a (24). The second step involves the selective hydrodechlorination of CFC-114a from the mixture to give HFC-134a and unreacted CFC-114 (25). Over a Pd-C catalyst, the yield to HFC-134a is >90%. A recent patent has claimed that CFC-114 can then be isomerized to CFC-114a, potentially giving a high overall three-step process (26). Numerous patent applications have claimed that a wide variety of metals can be used for the hydrodechlorination chemistry (Eq. 7), addressing problems associated with catalyst life (27).
Process Development
The recent patent literature has suggested that many of the routes indicated in Fig. 3 have received attention, and improvements can be expected. However, it is clear that the processes needed to manufacture alternatives will be far more complicated than existing CFC processes. In pilot plants around the world, chemists and engineers are aggressively working to develop critical data that will allow them to design and build world-scale commercial facilities in record time to meet the needs of society as CFCs are phased out. Obviously, for a development program of this magnitude and environmental significance, the normal time lines cannot be followed. Du Pont, for example, has ten pilot and interim commercial facilities to simultaneously develop process technology for many CFC alternatives and provide initial quantities for customer evaluations as well as toxicity testing. Commercial plants are being designed on the basis of a minimal amount of data, with the potential risk that a problem could occur during the pilot plant work that might require significant and costly changes. Data on the physical properties of many of the new compounds and their intermediates are not available, so extensive experimental work is required to make these measurements. Because many fluorocarbons form unpredictable azeotropes with each other and with HF, the purification operations of new plants can be expected to be complicated and to require extensive development.
International Cooperative Programs
Extensive toxicity studies will be required before large volumes of these new products will be released for general use. Because these studies are costly and timely, several CFC producers have formed consortia to share costs and expedite the testing process. To date, three consortia, PAFTs I, II, and III (Programs for Alternative Fluorocarbon Testing), have been established to evaluate the toxicity of HFC-134a and HFC-125 and HCFC-124, HCFC-123, and HCFC-141b. A report from PAFTs I and II indicated that no problems have been encountered, so far, for HFC-134a, HCFC 123, and HCFC-141b.
Another group, AFEAS (Alternative Fluorocarbon Environmental Acceptability Study), was formed to survey the effect of the alternatives and their degradation products on the environment. Their first report summarized existing data and proposed a program to collect the data needed to complete the evaluation. A $6-million 3-year program has been funded by 12 CFC producers, and work is under way to attempt to fill in the gaps where data are missing.
Remaining Key Issues
Action by the CFC producers and users around the world has been very positive. Many of the key programs are in place to provide a safe and timely transition away from CFCs to environmentally acceptable alternatives. Figure 4 shows the large improvements provided by HCFCs and HFCs in terms of ODP and halocarbon GWP. The scientific community has reached a consensus that the rapid and successful introduction of HFCs and HCFCs will result in lower stratospheric chlorine levels. Government cooperation is required to ensure a rapid transition away from CFCs. Uncertainty about the availability of HCFC and HFC alternatives delays commitments by producers of goods currently dependent on CFCs. Companies must know what alternatives will be available before committing major investment to the research and development necessary to use the new compounds in their products. Policymakers need to provide clear and prompt signals to industry and the consumer. If HCFCs and HFCs are deemed acceptable alternatives, a strong statement to that effect will speed their development and the transition from CFCs. If they are deemed unacceptable, industry may focus its resources elsewhere, which will likely mean continued use of CFCs until other viable technologies are identified.
The compatibility of CFC alternatives with the plastic parts found in many pieces of equipment is a concern. In many applications, parts such as the O-rings, gaskets, and seals will need to be replaced by other polymer materials that are not affected by the new materials. This can only occur after large quantities of CFC alternatives are available for extensive customer testing.
Energy efficiency must also be considered. In some refrigeration applications, the alternatives are less efficient than the CFCs they are replacing, which could result in higher energy consumption or require a significant redesign of the equipment.
Although many of the CFC-producing nations are likely to agree to a phaseout by the year 2000, they account for only about 30% of the world population. Developing countries must be encouraged to restrict their use of CFCs and implement new technology based on HFCs and HCFCs, to ensure a rapid and complete phaseout of CFCs.
The author is at the Central Research and Development Department, E. I. du Pont de Nemours & Company Experimental Station, Wilmington, DE 19880.
____________________
REFERENCES
1. F. Swarts, Bull. Acad R. Belg. 24, 309 (1892).
2. _____, ibid 26, 102 (1893).
3. T. Midgley and A. C. Henne, Ind. Eng. Chem. 22, 542 (1930).
4. J. P. Glas, Printed Circuit Assembly (10 to 19 September 1989).
5. F. S. Rowland, Am. Sci. 77, 36 (1989).
6. J. E. Lovelock, Nature 230, 379 (1971).
7. M. J. Molina and F. S. Rowland, ibid. 249, 810 (1974).
8. R. T. Watson, M. J. Prather, M. J. Kuryb, NASA Rg Publ. 1208 (1988).
9. Du Pont Alternatives Circular H07421 (August 1989).
10. M. O. McLinden and D. A. Didion, ASHRAE J. (December 1987), p. 32.
11. Du Pont Alternatives Circular H12524 (January 1989).
12. D. B. Bivens et al., SAE Tech. Pap. 890306 (1989); D. J. Bateman et al., SAE Tech. Pap. 900216 (1990).
13. ICI, U.S. Patent 3,755,477 (1973).
14. DuPont, U.S. Patent 2,230,925 (1941); DuPont, U.S. Patent 3,003,003 (1961).
15. Dow, U.S. Patent 2,885,427 (1959).
16. Kaiser, U.S. Patent 4,782,643 (1988).
17. A. K. Barbour, L. J. Belf, M. W. Buxton, Adv. Fluorine Chem. 3, 185 (1963).
18. ICI, U.S. Patent 4,129,603 (1978).
19. Daikin, Great Britain Patent 2,030,981 (1978).
20. Du Pont, E.P. Patent 328127 (1989).
21. ICI, U.S. Patent 4,158,675 (1979).
22. Du Pont, U.S. Patent 4,311,863 (1982); Mitsubishi Metal Japan Patent Application 1-228925 (1989).
23. Du Pont, U.S. Patent 4,851,595 (1989); ICI, E.P. Patent 300742 (1989).
24. M. Vecchio and G. Gropelli, J. Fluorine Chem. 4, 117 (1974); Montedison, Italian Patent 680,700 (1965).
25. ICI, Great Britain Patent 1,578,933 (1980); C. Gervasutti, L. Marangoni, W. Marra, J. Fluorine Chem. 19, 1 (1982).
26. Montedison, U.S. Patent 4,748,284 (1988).
27. Asahi Glass, Japanese Patent Applications 1-128942, 1-132537, and 1-132538 (1989).
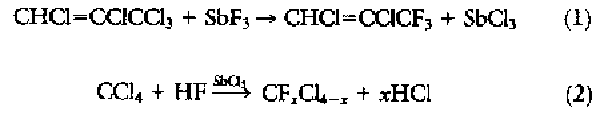 When Swarts later discovered
that the addition of trace quantities of pentavalent antimony as a "fluorine
carrier" allowed the reaction to be extended to a wide variety of chlorocarbons
(2), the era of commercially attractive fluorocarbon
chemistry began. The significance to modern society of fluorocarbons in refrigeration
systems, however, was not recognized until more than 30 years later.
When Swarts later discovered
that the addition of trace quantities of pentavalent antimony as a "fluorine
carrier" allowed the reaction to be extended to a wide variety of chlorocarbons
(2), the era of commercially attractive fluorocarbon
chemistry began. The significance to modern society of fluorocarbons in refrigeration
systems, however, was not recognized until more than 30 years later.
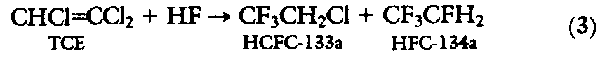 It appears unlikely that
this chemistry will provide a single-step, high-yield route to HFC-134a, analogous
to that for the preparation of CFC-12. Early work at Dow showed that the direct
reaction of TCE with HF gave only 3% HFC-134a and 78% HCFC-133a, with the use
of an "oxygenated chromium fluoride" catalyst at 300deg. to 400deg.C
It appears unlikely that
this chemistry will provide a single-step, high-yield route to HFC-134a, analogous
to that for the preparation of CFC-12. Early work at Dow showed that the direct
reaction of TCE with HF gave only 3% HFC-134a and 78% HCFC-133a, with the use
of an "oxygenated chromium fluoride" catalyst at 300deg. to 400deg.C