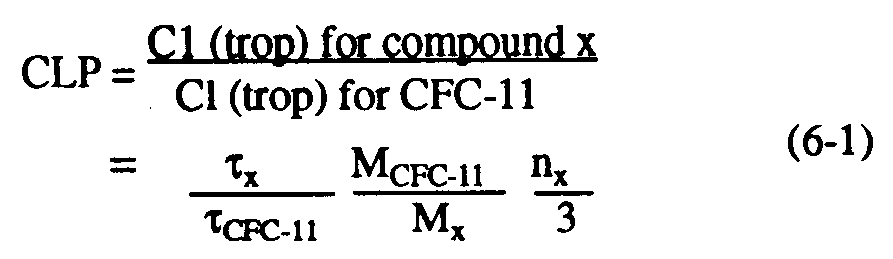
6.1 INTRODUCTION
Understanding of the depletion of stratospheric ozone by anthropogenic halocarbons has led to the need for simple measures for comparing the impact of one halocarbon source gas against others as a scientific guide to public policy. The simplest of these is the chlorine loading potential, or CLP. This index represents the amount of total chlorine delivered from the troposphere to the stratosphere due to emission of a given halocarbon, relative to that from a reference molecule, generally CFC-11 (see, e.g., Prather and Watson, 1990). CLPs can be considered upper limits to the steady-state relative effect on ozone depletion for most gases, and are defined by:
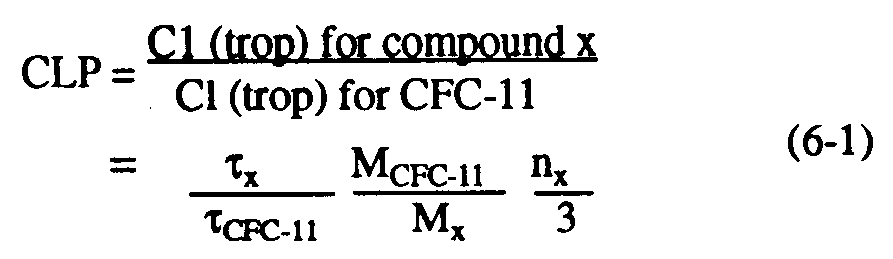
where Cl (trop) is the chlorine transported across the tropopause to the stratosphere per unit mass of halocarbon emitted; x,
CFC-11, MCFC-11 and Mx denote the lifetime and molecular weights of CFC-11 and the compound in question, respectively; and nx is the number of chlorine atoms per molecule of compound x. Similarly, the steady-state bromine loading potential (BLP) represents the total bromine delivered to the stratosphere relative to the chlorine due to CFC-11, and can be defined as:
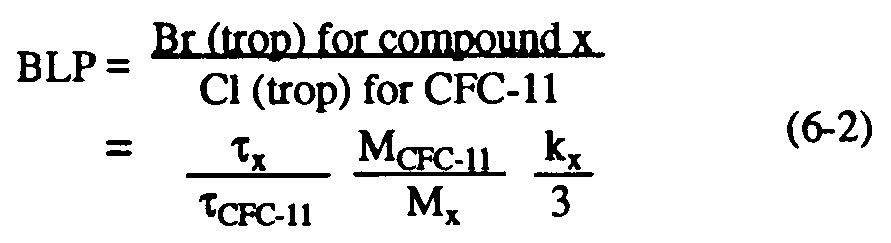
where kx is the number of bromine atoms per molecule of compound x. The chlorine loading for many HCFCs is determined largely by their decomposition within the troposphere through reaction with the reactive OH radical. Effective tropospheric OH concentrations can be estimated from observations of the trend in methyl chloroform together with information on its releases (e.g., Prinn et al., 1991). The lifetimes of other species similarly destroyed by reaction with tropospheric OH can then be inferred using laboratory measurements of their respective rate coefficients (e.g., Prather and Spivakovky, 1990). Estimates of the uncertainties in lifetimes and hence in CLPs will be discussed in Section 6.2.2.
Ozone depletion due to halocarbons depends not only upon the amount of chlorine or bromine delivered to the stratosphere from the troposphere (the CLP), but also on the following stratospheric processes: (i) the breakdown of the halocarbon within the stratosphere, which is responsible for liberating reactive halogen gases and (ii) the subsequent chemistry of the reactive halogens that controls the magnitude of the ozone depletion. Source gases with long stratospheric lifetimes will break down to release their chlorine or bromine less effectively than CFC-11, making their net impact on ozone smaller than their CLPs. It is important to distinguish between the tropospheric and stratospheric lifetimes, particularly for the HCFCs, which have relatively short tropospheric lifetimes but long stratospheric lifetimes (see Table 6-2). The need to consider stratospheric ozone loss processes in the assessment of relative impacts of halocarbons on the ozone layer has led to the use of numerical models for evaluation of the ozone depletion potential as first suggested by Wuebbles (1983). The ODP represents the amount of ozone destroyed by emission of a gas over its entire atmospheric lifetime (i.e., at steady state) relative to that due to emission of the same mass of CFC-11, and is defined in modeling calculations as follows:
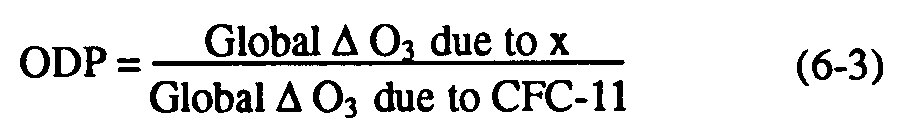
Because the ODP is a relative measure, it is likely that it can be calculated by present stratospheric models with greater reliability than the absolute ozone depletion, but it is important to quantify that reliability. The accuracy and reliability of ODP estimates will be addressed in this chapter by considering both statistical ranges of likely uncertainties and by examining observations.
The ODP/CLP ratio represents the efficiency of particular compounds for ozone destruction once in the stratosphere and allows for separation of the effects of stratospheric processes on the ozone depletion potential from those related to tropospheric removal and uncertainties in lifetimes. This will be discussed in more detail in Sections 6.2 and 6.3.
Two-dimensional models have become one of the principal tools for assessing the impact of anthropogenic emissions on the ozone layer. Such models generally include rather detailed representations of gas-phase photochemical processes and parameterized descriptions of stratospheric transport. These models have achieved significant successes in representing the chemical composition of the stratosphere, particularly at mid-latitudes above perhaps 25 km. However, recent research has demonstrated that current ozone depletion takes place largely below 25 km and is not well simulated by gas phase models (WMO, 1990; and Chapters 2 and 8 of this report). This occurs in part because current models do not contain satisfactory descriptions of the complicated microphysical processes governing the development of polar stratospheric clouds, the heterogenous reactions that occur on them, and the important processes of dehydration and denitrification, nor do they simulate in detail the transport processes that link polar and mid-latitude regions.
The breakdown of halocarbons within the stratosphere depends heavily upon both the modeled atmospheric transport and chemistry. It has been shown (e.g., Podolske et al., 1989) that current models fail to predict the very low concentrations of source gases and the correspondingly high concentrations of inorganic chlorine observed in the polar lower stratosphere in late winter. Without a realistic representation of the distributions of source gases and thus their chlorine (or bromine) release, the ODP cannot be calculated reliably even if the heterogeneous chemical processes leading to ozone depletion are considered in complete detail. The fact that the ODP is a relative measure alleviates, but does not eliminate, the role of shortcomings in model transport and ozone loss processes for modeling studies of ODPs, suggesting the need to examine observations to better constrain ODP estimates.
ODPs from gas phase model calculations were presented in WMO (1990) and by Fisher et al. (1990). Calculations by a variety of modeling groups were included. The general behavior, e.g., significantly higher ODPs for CFCs compared with HCFCs, was reproduced by all the models. However, there were also systematic differences between the models. For example, the Atmospheric and Environmental Research, Inc. (AER) two-dimensional model calculated somewhat smaller ODPs for the CFCs than the Oslo two-dimensional model, presumably a reflection of differences in stratospheric processes, whereas, for the HCFCs, the AER model calculated higher ODPs than the Oslo model, presumably a reflection of differences in tropospheric processes in the two models. These differences suggest that, for any particular model, the relative variation among ODPs of similar compounds (e.g., HCFCs) may be more accurately calculated than that for different types of compounds (e.g., a CFC versus an HCFC). Section 6.2 describes new calculations of ODPs and CLPs including some from models that consider heterogenous chemistry.
An alternative new approach to modeling studies of ODPs is to use observations of source gases to evaluate the chlorine or bromine release; such information coupled with observations of ozone loss can be used to deduce semi-empirical ODPs as described in Section 6.3. While subject to important uncertainties and assumptions, this semi-empirical method avoids the demanding requirements of accurate numerical simulation of source gas distributions and ozone destruction, and is therefore expected to be more realistic than present models, at least in the polar lower stratosphere where a significant fraction of contemporary ozone loss is found.
The time scale for the atmosphere to attain steady state with respect to injections of halocarbons ranges from a few years to many centuries, depending upon the lifetime of the species in question. Steady-state ODPs represent the best current understanding of the long-term impacts of such releases, but they do not describe short-term (decades or less) responses (WMO, 1990; SORG, 1990). When specific short-term time horizons are considered, time-dependent ODPs provide a more appropriate measure of impacts. Section 6.4 presents estimates of time-dependent ODPs for several halocarbons of interest over a range of time horizons.
6.2 MODEL CALCULATIONS OF CLPs AND ODPs
6.2.1 Compounds and Chemistry Considered
The list of compounds considered in this assessment (Table 6-1) is similar to that in WMO (1990), comprising most of the chlorofluorocarbons (CFCs) and other chlorinated halocarbons of interest, including several already being used or considered as replacements for CFCs, such as HCFC-22, HCFC-225ca, and HCFC-225cb. The list of halons considered here is much more extensive than the previous report, reflecting the increased interest in the environmental effects of these compounds. Since WMO (1990), the uncertainties in photolysis cross sections and reaction rate constants for many compounds have been narrowed considerably by improved laboratory studies. Table 6-1 shows the adopted kinetic parameters for the compounds evaluated in the Lawrence Livermore National Laboratory (LLNL) atmospheric models. It was not possible to ensure that all models presented here used the same kinetic parameters, but most of these differences are expected to be minor.
Detailed information is now available regarding the full temperature dependence of the ultraviolet (UV) and visible cross sections for many of the compounds examined, whereas models used in the WMO (1990) assessment were restricted to a single set of temperature-independent absorption coefficients for nearly all compounds. Inclusion of the temperature dependence can affect the altitude of Cl and Br release in the stratosphere. The temperature dependence of the absorption cross sections also affects the atmospheric lifetimes for several species, including H-1202, H-1211, and H-2402. Many of the bromoalkanes exhibit smooth continuum UV absorption cross sections in the region of 280-400 nm (near ultraviolet and visible). As pointed out by Burkholder et al. (1991), this region contributes significantly to atmospheric photoprocesses (because of the greatly increased transmitted flux there relative to shorter wavelengths) but the absolute absorption coefficients there are often small and difficult to measure. As an example, lifetimes ranging from 10 to greater than 50 years were calculated for H-1211 with the LLNL one-dimensional model, depending on the inclusion or exclusion of the temperature dependence of the cross sections and the extension of the long-wavelength tail, emphasizing the need for accurate measurements in this region.
Improved laboratory studies have changed the understanding of reaction rates with OH and hence the lifetimes and CLPs for several of the HCFCs. For example, reaction rates of OH with HCFC-141b (Talukdar et al., 1991b) and HCFC-142b (Gierczak et al., 1991) are about 30 percent and 15 percent smaller, respectively, than in the previous assessment.
All of the past calculations of ODPs were based on models including only gas phase chemistry and did not consider heterogeneous chemistry on sulfate aerosols or polar stratospheric cloud particles. In order to provide a historical perspective and because of limited time for preparing this report, most of the model results presented here are still based on gas phase chemistry. Some preliminary calculations are presented that attempt to consider heterogeneous processes (see Chapter 8).
Two-dimensional model calculations of atmospheric lifetimes, chlorine loading potentials, and ozone depletion potentials are included from five modeling groups: LLNL, AER, University of Oslo/Norsk Institute for Luftforskning, Norway (Oslo/NILU), and E.I. Du Pont de Nemours and Company (Du Pont). Most of the compounds have been evaluated with both the LLNL and AER models; the other groups evaluated a limited number of compounds.
6.2.2 Atmospheric Lifetimes and Chlorine Loading Potentials
Calculated lifetimes are shown in Table 6-2. The estimated lifetimes of the HCFCs are generally shorter than in the previous assessment, largely as a result of increases in calculated tropospheric hydroxyl concentrations due to new, more accurate measurements of the rate of the reaction of OH with CH, (Vaghjiani and Ravishankara, 1991). Differences of 20-30 percent are obtained for the calculated lifetimes for the HCFCs between the models used, reflecting the extreme difficulty and uncertainty associated with evaluation of tropospheric OH chemistry (see Chapter 5). Due to these problems, the lifetimes of compounds that react predominantly with OH are generally constrained by scaling to an assumed CH3CCl3 lifetime deduced from observations. In this chapter, lifetimes for all species in each model will be scaled to match the best estimates given in Chapter 8 and shown in Table 6-2. While such scaling imposes uniform CLPs, it fails to standardize other processes important to determining ODP values. For example, if different models calculate different tropospheric OH densities, then they are also likely to exhibit different stratospheric OH abundances. Stratospheric OH distributions have a minor impact on the lifetimes of the HCFCs, but control their stratospheric breakdown, subsequent chlorine release, and hence the ODP/CLP ratio. Similarly, many of the differences in calculated lifetimes for species destroyed by stratospheric photolysis (e.g., CFC-113) likely reflect differences in model transport and/or radiative transfer treatments that influence stratospheric chlorine release and ODP/CLP ratios, at least to some degree. These important model differences are not improved by standardizing the model lifetimes. In the following, ODP/CLP ratios will be taken directly from the individual model results, but the CLPs used to calculate ODPs will be based upon the standardized lifetimes.
A recent study using observations of CH3CCl3 together with estimates of its emissions suggests that the lifetime of CH3CCl3 may be shorter than previously thought due to changes in measurement calibrations (Prinn et al., 1992), while others have noted the possible importance of oceanic hydrolysis as a sink for this compound (Wine and Chameides, 1990; Butler et al., 1991). In contrast, many of the HCFCs are expected to undergo less oceanic hydrolysis, so that the fraction of their total lifetime attributable to loss with OH may differ from that of methyl chloroform. These considerations imply that the use of methyl chloroform to deduce an effective average tropospheric OH concentration is less certain than previously thought, with attendant uncertainties for estimates of CLPs and ODPs for compounds destroyed by reaction with OH. The lifetimes of compounds destroyed by stratospheric photolysis (including the reference molecule for ODP calculations, CFC-11) also have significant uncertainties. These are due in part to uncertainties in the dynamical processes responsible for transporting these compounds to the altitudes where their chemical destruction is rapid. The range of model values shown in Table 6-2 is likely to be representative of these uncertainties.
The range of total uncertainty in chlorine loading potentials for some species is illustrated explicitly in Figure 6-1, wherein estimated accuracies were used to deduce cumulative probability distribution functions (e.g., Lindgren, 1968). The uncertainties considered are indicated on the figure. This approach assumes that these errors are randomly distributed and is intended only to illustrate the current best estimate of the range of statistically probable error. The figure illustrates the fact that uncertainties in steady-state CLPs can be large for some compounds, and that the CLPs for HCFC-123, HCFC-22, and HCFC-141b are likely (>90 percent probability) to lie below 0.035, 0.26, and 0.29, respectively.
Additional uncertainties exist, but most of these are related to fundamental understanding rather than numerical accuracy and are not included in Figure 6-1. In deriving the CLPs shown in Figure 6-1 and Table 6-2, it has been assumed that the products of all decomposition reactions in the troposphere are too short-lived to reach the stratosphere. However, some HCFCs (e.g., HCFC-141b and 142b) likely produce halogen-containing acyl peroxynitrates as products of their oxidation with tropospheric OH (see Chapter 5). If these peroxynitrates are sufficiently long-lived to be transported to the stratosphere to some degree, then an effective increase in CLPs will result. Further, it is possible that OH and/or HO2 radicals may be removed on aerosol surfaces in the troposphere and/or stratosphere, affecting the lifetimes of compounds removed by tropospheric OH and perhaps their stratospheric chlorine release. Although observations of CH3CCl3 place some constraints on the magnitude of tropospheric OH in an averaged sense and thus upon such reactions, they cannot be ruled out. Further, CLPs represent best estimates for the contemporary atmosphere but neglect possible changes that may occur over the lifetimes of the compounds in question. For example, future changes in tropospheric water vapor and hence OH concentrations (due, for example, to atmospheric climatic change) would affect the CLPs of many compounds but are not considered here.
6.2.3 Calculated ODPs
Table 6-3 presents ODPs for CFCs, HCFCs, halons and other halocarbons calculated with the two-dimensional models considered. The model-calculated ODPs for CH3CCl3 and the HCFCs are generally less than 0.15, while those for the CFCs and halons are much larger. Chlorinated compounds with stratospheric lifetimes comparable to or shorter than that of CFC-11 display ODP/CLP ratios close to 1, reflecting the fact that they release their chlorine in a manner similar to the reference gas and hence have similar impacts on ozone once in the stratosphere. As mentioned in Section 6.1, chlorinated compounds with stratospheric lifetimes longer than that of CFC-11 release their chlorine more slowly and display ODP/CLP ratios that are less than 1. Many of the HCFCs have relatively small ODP/CLP values due to their slow stratospheric breakdown by reaction with OH (see Table 6-2). Clearly, the least damaging chlorinated substances are those that are destroyed rapidly within the troposphere (thus limiting their CLPs) but slowly in the stratosphere (reducing the ODP/CLP ratio).
For the halons, the ODP/BLP ratios depend on the efficiency of bromine as compared to chlorine in destroying ozone as well as on the stratospheric lifetime (hence the bromine release). The efficiency of bromine relative to chlorine in depleting ozone is hereafter referred to as , and is expected to be close to the ODP/BLP ratio for all of the halons considered here. It is important to note that
is dependent on the absolute amount of reactive chlorine, due to chemical coupling between chlorine and bromine that controls the impact of bromine on ozone. The
values given here pertain to conditions of total reactive chlorine loading close to contemporary levels. The scenarios presented in Chapter 8 suggest that the future chlorine loading is likely to change by less than 20 percent for at least the next several decades. The calculated globally-averaged
values suggested by the ODP/BLP ratios in Table 6-3 range from about 10 to 80. These illustrate the extreme effectiveness of bromine in catalyzing ozone destruction (first noted by Yung et al., 1980). The model-calculated
values for the halons depend critically on the portion of bromine species found in the active form (BrO), which in turn depends on the formation and removal rates of the reservoir species, HBr and BrONO2. Some measurements of HBr appear difficult to reconcile with present theory (Park et al., 1989). However, measurements of BrO by Brune et al. (1988) are in general agreement with model predictions, lending some support to the calculated
values and current photochemical schemes for bromine. The precise values of
for mid-latitudes also depend on calculated concentrations of atomic oxygen, OH, and HO2 in the lower stratosphere, which are subject to considerable uncertainties. The values reported in the table should therefore be considered as preliminary. Note, however, that the value of
in the polar lower stratosphere is much better understood as discussed later (see Section 6.3).
Most of the calculations include gas phase chemistry only, but some calculations have also been carried out with the AER, LLNL, and Du Pont models, including a parameterization of known heterogeneous chemistry on sulfate aerosols, and with a treatment of PSC chemistry for the Oslo model (see Chapter 8). The ODP/CLP and ODP/BLP ratios calculated with heterogeneous chemistry in Table 6-3 are very similar to those from the gas phase models presented here for nearly all species. However, as discussed in more detail below and in Chapter 8, the models including heterogeneous chemistry still do not fully describe the chlorine release nor the ozone loss observed in the lower stratosphere at mid and high latitudes, so that their calculated ODPs remain subject to uncertainties.
6.3 COMPARISON OF MODELED ODPs AND INFERENCES FROM OBSERVATIONS
In this section, semi-empirical information useful for estimating the ratio between ODP and CLP will be briefly described, the results presented, and compared to the models used here. Briefly, the approach used (Solomon et al., 1992) consists of using direct measurements of halocarbons to evaluate chlorine release and hence local ODP/CLP ratios, particularly in the polar lower stratosphere. When direct measurements are limited, correlations are used to infer abundances for some halocarbons from those of other tracers and hence their ODP/CLP ratios. Globally-averaged ODP/CLP ratios are estimated for some compounds using local ODP/CLP values together with an observationally-derived ozone loss distribution assuming that the ozone loss is due only to halogen chemistry.
The fraction of halocarbon dissociated within the stratosphere (F) can be defined as:
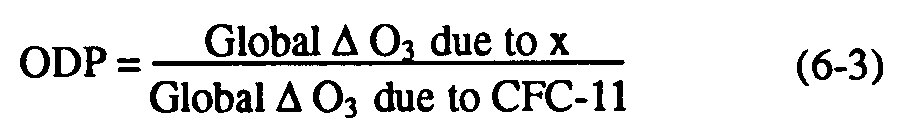
where entry, denotes the mixing ratio of halocarbon entering the stratosphere and
,z indicates the mixing ratio at any latitude and altitude. Measurements of the distributions of halocarbons within the stratosphere can be used to define
,z. Since the tropical troposphere is rather well-mixed for species with lifetimes longer than a few months,
entry is likely to be nearly equal to the surface mixing ratio. Time lags for transport between the troposphere and stratosphere influence the value of uentry for any molecule whose abundance is changing with time (e.g., HCFC-22, CFC-11). Such lags are believed to be short (2 years or less) over much of the atmosphere, but measurements of CO2 suggest time lags of about 3-5 years in the polar lower stratosphere (Heidt et al., 1991; Schmidt and Khedim, 1991).
As noted in WMO (1990), the local ozone depletion in regions of rapid chlorine-catalyzed destruction of ozone is proportional to the relative stratospheric chlorine release (Clrel) per unit of surface emission of substance, which can be defined as the ratio of fractional dissociation of a particular molecule to that of CFC-11, multiplied by the chlorine loading potential. This implies that the local ozone depletion potential [ODP(,z)] at any point can be defined as follows (see Solomon et al., 1992):
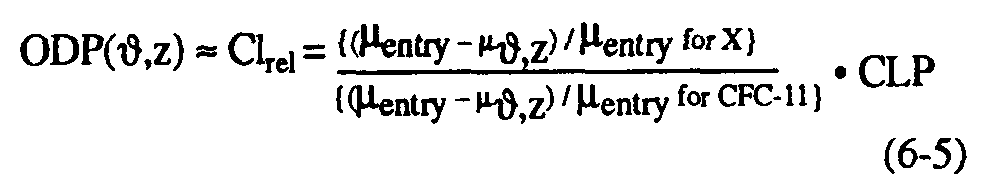
Equation (6-5) allows estimation of the local ODP/CLP ratio from available measurements of CFC-11 together with other halocarbons. This approach has been taken for a number of halocarbons of interest, particularly HCFC-22, CCl4, CFC-113, and CH3CCl3 in polar regions (Solomon et al., 1992). Simultaneous airborne measurements of these species together with CFC-11 were obtained by Heidt and coworkers (see, Heidt et al., 1989) during the Airborne Arctic Stratospheric Expedition (AASE) in January-February 1989. The polar measurements of CH4, N2O, and CFC-11 used here have been stringently intercompared with other calibration standards and are believed to be accurate to within a few percent. Because of the large ozone losses in the Arctic and Antarctic as described in Chapter 2, the local ozone depletion potentials of the polar lower stratospheres are of considerable importance in determining the globally-averaged ODP as well as in understanding requirements for reducing or eliminating ozone losses such as the Antarctic ozone hole. Table 6-4 presents Arctic lower stratospheric local ozone depletion potentials from these direct measurements, averaged over the region from 15-21 km; similar numbers have been obtained from Antarctic lower stratospheric measurements obtained in September 1987. Growth rates for these source gases have been taken from WMO (1990) and results are shown for several cases assuming no time lag between the troposphere and stratosphere and assuming a 3-year lag as discussed earlier.
Direct observations of HCFCs and halons are, however, extremely limited for many species. In the absence of direct information, correlations among long-lived tracers provide a useful means to infer the distribution of one compound from observations of another, thus extending the possible range in space and in compounds evaluated (see Fahey et al., 1990; Solomon et al., 1992; Plumb and Ko, 1991). Plumb and Ko (1991) have shown that the correlations between long-lived tracers are likely to be robust parameters that depend largely on the ratio of stratospheric loss rates and not upon the details of transport processes. The correlations between species can therefore be calculated in models with far greater confidence than the absolute tracer abundances or ODPs. In the following, abundances of several HCFCs and their corresponding local ODPs are inferred from observations of CH4 or N2O using correlations from model calculations (see Solomon et al., 1992 for details). Table 6-4 shows that the polar ODP inferred for HCFC-22 using an assumed correlation with CH4 gave results close to that obtained with direct measurements of HCFC-22 when a 3-year lag time was assumed. The calibration standard used here for HCFC-22 has not yet been intercompared in detail with others. This comparison nonetheless strengthens confidence in the correlation approach. Further, it is important to note that the semi-empirical ODP deduced from direct measurements of HCFC-22 is sensitive to the assumed age of the air due to the rapid (order 7-10 percent per year) rate of increase of this compound in the contemporary atmosphere, while the values derived from CH4 display little dependence on the assumed age since the CH4 growth rate is only about 0.7 percent per year.
Figure 6-2 presents the local chlorine release profile inferred for mid-latitude winter for HCFC-22 (based upon measurements of CFC-11 and CH4 together with an adopted correlation between CH4 and HCFC-22 that is in good agreement with available direct measurements as described in Solomon et al., 1992). Values taken from several models used in this assessment are shown for comparison. Because of the possibility of sharp latitude gradients, detailed comparisons between models and measurements should be carried out as a function of latitude as well as altitude, so that Figure 6-2 provides only general guides to the comparisons between models, and between models and quantities inferred from measurements. Figure 6-2 reveals overall consistency between the shapes of the vertical profiles of the modeled and inferred relative chlorine release but many differences in detail. The discrepancies in the lower stratosphere where much of present-day ozone loss occurs (see Chapter 2) are of the greatest importance to estimates of ODPs.
Comparison of Tables 6-3 and 6-4 shows that the observationally-based polar lower stratospheric ODP/CLP ratios are generally larger than the globally-averaged values obtained in gas phase models for chlorocarbons whose stratospheric lifetimes are longer than that of CFC-11 (e.g., HCFC-22 and HCFC-142b), while the observationally-based values tend to be slightly smaller than the model calculations for chlorocarbons that exhibit stratospheric loss time scales that are shorter than that of CFC-11 (e.g., CCl4). This primarily reflects differences in the time-dependent breakup of these molecules within the stratosphere relative to CFC-11, coupled with the fact that the air within the polar lower stratosphere is likely to be relatively 'old' compared to other regions where much of the modeled ozone depletion takes place.
The extrapolation of the local approach to the global scale requires empirically-based specifications of the global distributions of both long-lived tracers and ozone loss. A global semi-empirical ozone depletion potential may be defined (Solomon et al., 1992) as:
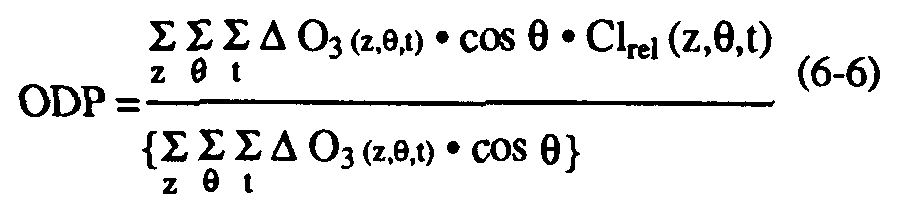
where O3 (z,
,t) is the estimated decrease in ozone concentration observed in today's atmosphere at each altitude, latitude, and time of year (see Solomon et al., 1992 for details of the ozone loss distribution imposed). This equation involves the important assumptions that all of the observed ozone depletion in the contemporary atmosphere is due to halogen chemistry and that the depletion is proportional to the local relative chlorine and/or bromine release (see also, Solomon et al., 1992). These assumptions are well justified in polar regions and likely at mid-latitudes.
Table 6-4 presents semi-empirical global ODPs for the following compounds: HCFC-22, HCFC-142b, and CCl4. A range of values is presented, showing the effects obtained if it is assumed (as extreme cases) that the polar vortex acts as a processor (i.e., assuming that mid-latitude lower stratospheric ozone losses have their origin entirely in the polar region) and assuming that the polar vortex is completely isolated (see the discussion in Chapter 4 of this assessment). The global ODP/CLP ratios inferred are dominated by ozone losses in the lower stratosphere and are similar to those obtained from the direct Arctic measurements. Thus the polar ODPs are likely to be close approximations to the global values.
Observations can also be used to place constraints on local ozone depletion potentials for halons in the polar regions. The lifetimes of most halons are rather short within the stratosphere due to rapid photolysis (see Table 6-2), and grab-sampling measurements of, for example, Halon-1211 during AASE suggest that it is nearly completely dissociated in the Arctic over the height range from about 15 to 21 km (W. Pollock and L. Heidt, personal communication, 1991). Assuming that the halons will be dissociated by at least as large a fraction compared to their input values to the stratosphere as CFC-11 in the polar lower stratosphere, the ozone depletion potentials for halons (or BODP) in the contemporary stratosphere can be defined as:

Direct measurements of BrO, ClO, and OClO in the polar stratosphere confirm the role of bromine in polar ozone loss and constrain the value of there considerably better than the global estimates from models discussed earlier. These show that it is at least 40 in this region (see Chapter 8 and references in Solomon et al., 1992). A value of 40 is thus adopted here.
Table 6-5 presents the semi-empirical ODPs inferred from equation 6-7 for the halons, compares the semi-empirical and modeled ODPs for all compounds, and presents the recommended ODPs for this assessment, based mainly on the semi-empirical approach with guidance from the models. Semi-empirical ODPs for HCFC-123, HCFC-124, HCFC-225ca, and HCFC-225cb were obtained using the correlation between these molecules and CH4 from the LLNL model, using the approach of Solomon et al. (1992). The ODPs for the halons derived both semi-empirically and from some models shown in Table 6-3 are considerably larger than the estimates of the previous assessment. The semi-empirical ODP/CLP values for HCFC-22 are larger than the range of the models shown here by about 20 to 80 percent, while for HCFC-142b, the inferred values are as much as a factor of two greater than some model values. For all of the HCFCs considered here, the inferred ODP/CLP values are, however, far below one, showing that the upper limit represented by the CLPs is not realized.
The uncertainty in the semi-empirical polar ODP/CLP ratios for most species considered is mainly dependent on the relative calibration of the tracer measurements and is estimated to be on the order of +/- 10 percent for compounds inferred from CH4 measurements. Combining this uncertainty with that of the CLPs discussed earlier allows estimation of the statistically probable error range for ODP values. Figure 6-3 presents the cumulative probability distribution functions for the ODPs of several molecules of interest based upon the known uncertainties considered. The figure shows that current uncertainties suggest that the ODP is highly likely (>90 percent) to lie below 0.04 for HCFC-123, below 0.11 for HCFC-22, and below 0.23 for HCFC-141b. However, it should be emphasized that these ranges are likely to be underestimates since they are based purely on statistical errors in present understanding and do not include chemical and physical processes whose influences could be large but cannot currently be quantified (see Section 6.2.2).
6.4 TIME DEPENDENT EFFECTS
The ODP of compound x, defined in Section 6.1, refers to conditions when the two compounds, x and CFC-11, have reached their steady-state concentrations. Consider the example of compound x, an HCFC with a relatively short tropospheric lifetime. The steady-state ODP compares the calculated steady-state ozone loss due to x, which will be realized within perhaps a decade or so, with the ozone loss due to CFC-11, realized on the time scale of centuries. On the other hand, the ratio of the ozone loss due to compound x compared to that from CFC-11, if calculated after about a decade, will be much larger than the steady-state ODP of compound x. All of x's ozone depletion has already occurred while the CFC-11 depletion is still increasing. Thus, while the ODPs for some short-lived compounds, including the HCFCs, may suggest only a modest impact on the chronic response by ozone, the acute response over the next several years will be more substantial.
As in estimations of greenhouse warming potentials (see Chapter 7), relative impacts can be assessed not just for steady state, but also for specific time horizons. The time-dependent, semi-empirical ODP can be defined as follows (see Solomon and Albritton, 1992):
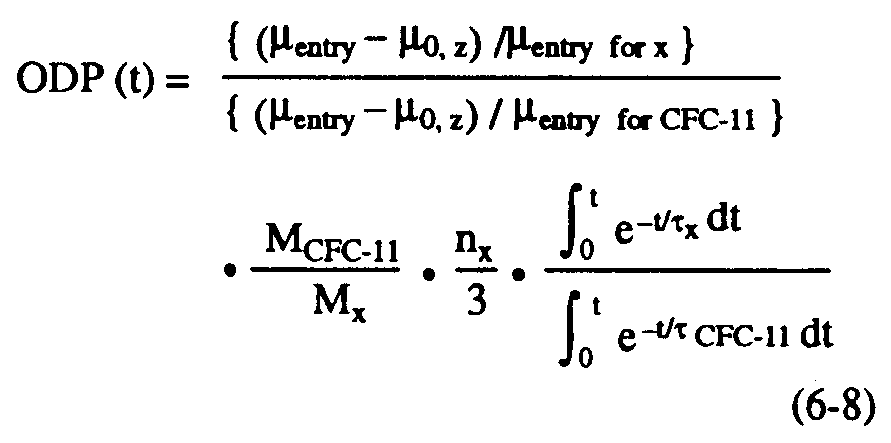
where t is the time horizon and the other symbols have all been defined previously. The terms in brackets represent the fractional dissociation of x and CFC-11, respectively (equation 6-4) in the region considered. As in the previous section, these will be evaluated below using polar semi-empirical estimates based on observations. In principle, time lags for transport to the stratosphere could be considered in evaluating ODPs over specific time horizons but these are not expected to change the results greatly and were neglected for simplicity in the calculations presented here.
Figure 6-4 shows polar lower stratospheric ODPs for several HCFCs and halons as a function of time horizon using the CLPs of Table 6-2. The large ODPs for bromine-containing compounds reflect the large value of . Compounds with relatively short lifetimes display reduced impacts over long time scales but large ODPs over short time horizons. Note that although CH3Br displays a relatively small ODP over long time horizons, it has a very large ODP for short time scales by virtue of its short lifetime and the large value of
. The large ODPs for CH3CCl3 obtained over short time horizons reflect the very short lifetimes of this gas, allowing for large and rapid impacts on ozone. The HCFCs have relatively short lifetimes, and generally display small ODPs over long time horizons, but exhibit ODPs over short time scales that can be as much as 3 to 10 times greater than their steady-state ODPs. The opposite is true for compounds with lifetimes comparable to or longer than that of CFC-11 (e.g., CFC-113), which display smaller ODPs over short time horizons than they do over time scales of centuries.
The calculations presented here illustrate the roles of various compounds in depleting ozone both over the long and short term. Likely future changes in atmospheric chlorine and bromine are described in Chapter 8, where various emission scenarios are considered. Current restrictions on emissions of halocarbons suggest that the chlorine content of the stratosphere will continue to increase for at least the next decade, implying that further ozone reductions are likely in the near future. During this period, ODPs for the specific time horizon in question are expected to best represent the anticipated relative ozone response to releases of various compounds.
REFERENCES
Braun, W., A. Fahr, R. Klein, M.J. Kurylo, and R.E. Huie, UV gas and liquid phase absorption cross section measurements of hydrochlorofluorocarbons HCFC-225ca and HCFC-225cb, J. Geophys. Res., 96, 13009-13015, 1991.
Brown, A.C., C.E. Canosa-Mas, A.D. Parr, J.M.T. Pierce, and R.P. Wayne, Tropospheric lifetimes of halogenated anaesthetics, Nature, 341, 635-637, 1989.
Brune, W.H., D.W. Toohey, J.G. Anderson, W.L. Starr, J.F. Vedder, and E.F. Danielson, in situ northern mid-latitude observations of ClO, O3, and BrO in the wintertime lower stratosphere, Science, 242, 558-562, 1988.
Burkholder, J.B., R.R. Wilson, T. Gierczak, R. Talukdar, S.A. McKeen, J.J. Orlando, G.L. Vaghjiani, and A.R. Ravishankara, Atmospheric fate of CF3Br, CF2Br2, CF2ClBr, and CF2BrCF2Br, J. Geophys. Res., 96, 5025-5043, 1991.
Butler, J.H., J.W. Elkins, T.M. Thompson, B.D. Hall, T.H. Swanson, and V. Koropalov, Oceanic consumption of CH3CCl3: Implications for tropospheric OH, J. Geophys. Res., 96, 22347-22355, 1991.
Fahey, D.W., S. Solomon, S.R. Kawa, M. Loewenstein, J.R. Podolske, S.E. Strahan, and K.R. Chan, Reactive nitrogen and nitrous oxide in the lower polar stratosphere: a case study in the coupling of photochemistry and dynamics, Nature, 345, 698, 1990.
Fisher, D.A., C.H. Hales, D.L. Filkin, M.K. W. Ko, N.D. Sze, P.S. Connell, D.J. Wuebbles, I.S.A. Isaksen, and F. Stordal, Model calculations of the relative effects of CFCs and their replacements on stratospheric ozone, Nature, 344, 508, 1990.
Gierczak, T.,R. Talukdar, G.L. Vaghjiani, E.R. Lovejoy and A.R. Ravishankara, Atmospheric chemistry of hydrofluoroethanes and hydrochlorofluoroethanes: 1. Rate coefficients for reactions with OH, J. Geophys. Res., 96, 5001, 1991.
Gillotay, D. and P.C. Simon, Ultraviolet absorption cross sections of photoactive species of stratospheric interest, Part 1: The Halocarbons, Aeronomica Acta A, No. 356, Institut D'Aeronomic Spatiale De Belgique, 173, 1990.
Gillotay, D. and P.C. Simon, Temperature-dependence of ultraviolet absorption cross sections of alternative chlorofluoroethanes, J. Atm. Chem., 12, 269-285, 1991.
Heidt, L.E., J.F. Vedder, W.H. Pollock, R.A. Lueb, and B.E. Henry, Trace gases in the Antarctic atmosphere, J. Geophys. Res., 94, 11599, 1989.
Heidt, L.E., S.J. Hovde, A.F. Tuck, J. Vedder, and R. Weiss, The age of the air in the Arctic lower stratospheric vortex during late winter 1988/9, in preparation for submission to J. Geophys. Res., 1991.
JPL 90-1, Chemical kinetics and photochemical data for use in stratospheric modeling, NASA Panel for Data Evaluation, evaluation number 9, JPL Publ. 90-1, 1990.
Lindgren, B.W., Stalistical theory, McMillan Press, New York, 1968.
Orkin, V.L., V.G. Khamaganov, and E.E. Kasimobskaya, Investigation of elementary processes which determine the Ozone Depletion Potential of some halogenated hydrocarbons, 11th International Symposium on Gas Kinetics, Assissi, Italy, 1990.
Orlando, J.J., J.B. Burkholder, S.A. McKeen, and A.R. Ravishankara, Atmospheric fate of several hydrofluoroethanes and hydrochloroethanes: 2. UV absorption cross sections and atmospheric lifetimes, J. Geophys. Res., 96, 5013-5023, 1991.
Park, J.H., B. Carli, and A. Barbis, Stratospheric HBr mixing ratio obtained from far infrared emission spectra, Geophys. Res. Let., 16, 787-790, 1989.
Plumb, R.A., and M.K. W. Ko, Interrelationships between mixing ratios of long-lived stratospheric constituents, submitted to J. Geophys. Res., 1991.
Podolske, J.R., M. Loewenstein, S.E. Strahan, and K.R. Chan, Stratospheric nitrous oxide distribution in the Southern Hemisphere, J. Geophys. Res., 94, 16767, 1989.
Prather, M.J., and C.M. Spivakovsky, Tropospheric OH and the lifetimes of hydrochlorofluorocarbons (HCFCs), J. Geophys. Res, 95, 18723-18729, 1990.
Prather, M.J., and R.T. Watson, Stratospheric ozone depletion and future levels of atmospheric chlorine and bromine, Nature, 344, 729-734, 1990.
Prinn, R., D. Cunnold, P. Simmonds, F. Alyea, R. Boldi, A. Crawford, P. Fraser, D. Gutzler, D. Hartley, R. Rosen, and R. Rasmussen, Global average concentration and trend for hydroxyl radicals deduced from ALE/GAGE trichloroethane (methyl chloroform) data for 1978-1990, J. Geophys. Res., 97, 2445-2462, 1992.
Schmidt, U., and A. Khedim, In-situ measurements of carbon dioxide in the winter arctic vortex and at mid-latitudes: an indicator of the 'age' of stratospheric air, Geophys. Res. Lett., 18, 763-766, 1991.
Solomon, S., M. Mills, L.E. Heidt, W.H. Pollock, and A.F. Tuck, On the evaluation of ozone depletion potentials, J. Geophys. Res., 97, 825-842, 1992.
Solomon, S., and D.L. Albritton, A new analysis of time-dependent ozone depletion potentials, submitted to Nature, 1992.
SORG (U. K. Stratospheric Ozone Review Group), Stratospheric Ozone 1990, Crown Copyright, 1990.
Talukdar, R., A. Mellouki, T. Gierczak, J.B. Burkholder, S.A. McKeen, and A..R. Ravishankara, Atmospheric lifetime of CHF2Br, a proposed substitute for halons, Science, 252, 693-695, 1991a
Talukdar, R., A. Mellouki, T. Gierczak, J.B. Burkholder, S.A. McKeen, and A..R. Ravishankara, Atmospheric fate of CF2H2, CH2CF3, CHF2CF3, and CH3CFCl2: Rate coefficients for reactions with OH and UV absorption cross sections of CH3CFCl2, J. Phys. Chem., 95, 5815-5821, 1991b.
Vaghjiani, G.L., and A.R. Ravishankala, New measurement of the rate coefficient for the reaction of OH with methane, Nature, 350, 406-409, 1991.
Vanlaethem-Meuree, N., J. Wisemberg, and P.C. Simon, Ultraviolet absorption spectrum of methylchloroform in the vapor phase, Geophys. Res. Lett., 6, 451-454, 1979.
Wine, P.H., and W.L. Chameides, Possible atmospheric lifetimes and chemical reaction mechanisms for selected HCFCs, HFCs, CH3CCl3 and their degradation products against dissolution and/or degradation in seawater and cloudwater, in Scientific assessment of stratospheric ozone: 1989, World Meteorological Organization, report number 20, volume 2, p. 269, 1990.
WMO, Scientifc assessment of stratospheric ozone: 1989, World Meteorological Organization, report number 20, vols. 1 and 2, 1990.
Wuebbles, D.J., Chlorocarbon emission scenarios: potential impact on stratospheric ozone, J. Geophys. Res., 88, 1433, 1983.
Yung, Y.L., J.P. Pinto, R.T. Watson, and S.P. Sander, Atmospheric bromine and ozone perturbations in the lower stratosphere, J. Atmos. Sci., 37, 339, 1980.